As the territory of the Roman Empire spread across the Mediterranean Sea, the Romans obtained more regions containing new resources. One of these regions was the island of Cyprus, which contained a mine from which a reddish metal was extracted.
The Romans called this metal aes cyprium, which is Latin for metal of Cyprus. As time went on, the phrase was corrupted and the metal was referred to as cuprum. Cuprum was eventually anglicized and added to the Old English vocabulary as coper, and around the year 1530, its English name became copper.

Copper was given the element symbol Cu in honor of the Latin name of the metal. The cations of copper were also named in honor of cuprum: cupric and cuprous. Because copper is a transition metal, it can have more than one oxidation state. Unlike strontium, which can only have an oxidation state of +2, copper can have the oxidation states of +1 (cuprous), +2 (cupric), and +3, however, the +3 oxidation state is quite rare. Cuprous was given its name by adding the -ous ending (denoting a lower charge) to cuprum and cupric was named by placing the -ic ending (denoting a higher charge) on cuprum.
The oxidation state determines how many electrons an ion wishes to share with another ion and it is represented either by Roman numerals within parentheses or the -ous and -ic endings. For example, the copper in Cu2O has an oxidation state of +1, therefore, the compound is either called Copper (I) Oxide or Cuprous Oxide; the copper in CuO has an oxidation state of +2, therefore, the compound is either called Copper (II) Oxide or Cupric Oxide.
Copper Salt Colors
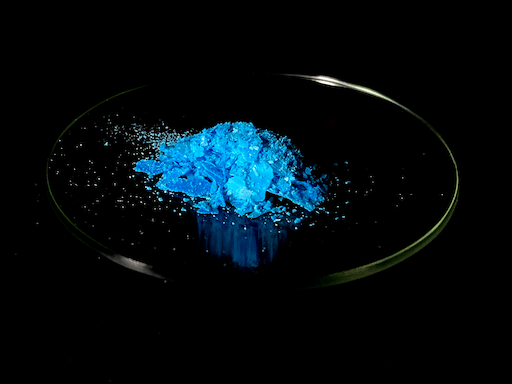
Many of the salts of copper are colored, which is typical of transition metals. Besides a few exception, such as the red color of Copper (I) Oxide and the black color of Copper (II) Oxide, most of copper’s compounds are either blue or green.
An interesting fact that I realized was that copper, a reddish metal, has many salts that are blue-green and green. What makes this fascinating is that red and green are opposites on the color wheel, in other words, the color of copper flips to the other side of the color wheel once it becomes an ion.
In my experience, I found Copper (II) Chloride to be one of the strangest copper salts; when the salt was in solution and fully hydrated, it was a pastel blue, but as I evaporated the water, the salt turned green. Once I isolated the salt, I dehydrated it almost completely, which caused it to become a yellowish brown.
Copper Salts
There are many interesting copper salts. I happened to have several copper salts and the ingredients to make several others on hand. Here is a quick list of some copper salts.




Making Copper Salts
WARNING: do not replicate the chemical reactions featured in this post! Not only are the reactants and products harmful to plant, animal, and human life, but copper salts can easily stain surfaces and skin. Copper (II) Chloride is also known to violently attack and destroy aluminum.
In order to make Copper (II) Chloride, I wrote the following chemical equation.
CuSO4 + CaCl2 -> CuCl2 + CaSO4
This seemed pretty straightforward initially, however, I remembered the mistake of not accounting for the water in the chemicals’ crystals that I made during my strontium reaction. I could use this chemical equation, but because many salts have water in their crystal structures — which causes the chemicals to weigh more — my measurements would be inaccurate. For that reason, I was forced to take the water into account with the following chemical equation:
CuSO4 • 5 H2O + CaCl2 • 2 H2O -> CuCl2 • 2 H2O + CaSO4 • 2 H2O + 3 H2O
I also made Copper (II) Nitrate using the following equation:
CuSO4 • 5 H2O + Sr(NO3)2 • 4 H2O -> Cu(NO3)2 • 3 H2O + SrSO4 • 1/2 H2O + 5 1/2 H2O
Here is a video of my making the Cupric Carbonate and the Cupric Chloride and a video of a flame test using my Cupric Chloride.
Interestingly, the Cupric Chloride burned green with flashes of intense blue. This happens because copper usually burns with a green flame, but in the presence of chlorine (the chloride ion from Cupric Chloride), the copper burns with a blue flame.
What do you like most about copper?

