Though I own element cubes from Luciteria, I don’t entirely feel satisfied with them. I attempted to make cubes from relatively pure samples of several elements, such as aluminum, lead, and sulfur in previous posts.
While staring intently at my homemade element cubes, I resolved to make as many element cubes as I could practically and safely produce, once I gain access to a sample of the element that is not so valuable that I’m unwilling to destroy it.
I contemplated the elements which I had yet to turn into cube form — which is still the vast majority of them — and I realized that there is one element that I haven’t made into a cube yet, which I handle and consume daily: carbon.
Some of the greatest attributes of carbon are that it is cheap and it is everywhere; all living organisms contain it, including the foods that you eat, except for a couple of inorganic molecules such as salt and water.
Making the Carbon Cube
All I have to do to make a carbon cube is acquire one of the allotropes of carbon. Allotropes are different physical structures of the same, pure element. Most pure elements only exist in one form, but five elements have allotropes: phosphorus, sulfur, oxygen, tin, and carbon.
Elemental carbon can be found in many different allotropes, but three of the most common allotropes are graphite, diamond, and buckminsterfullerene, however, turning these allotropes into cubes isn’t something that I can currently do. At this point in time, I don’t have any graphite that I am willing to cut into a cube; a pure diamond cube would be amazing, but far too expensive; and I don’t have the capabilities of purifying buckminsterfullerene from soot — truthfully, I didn’t know that buckminsterfullerene existed until now.
So how did I make a carbon cube if I didn’t use any of carbon’s common allotropes? I simply cut a cube out of the amorphous allotropic form of carbon that I made from scratch. All right, that’s just a fancy way of saying that I made a cube from charcoal. Charcoal is an amorphous form of carbon, which means that it does not have a crystalline structure like diamond and graphite.
The benefit of using charcoal is that it is astonishingly easy to make, in fact, you could technically make this charcoal element cube in any forest, even without tools; if you don’t have access to trees, virtually any plant material could also be turned into charcoal, but it may not be as structurally sound as wood charcoal.
I make charcoal all the time for pyrotechnics by simply heating some wood in a sealed container with a small vent. When wood is brought to a high temperature, most of the components of the wood vaporize (which is observed as smoke), leaving behind a spongy, amorphous carbon structure which was once bonded with hydrogen and oxygen in molecules such as cellulose, hemicellulose, and lignin; this amorphous carbon structure is usually called charcoal.
When the wood is heated in the air, the hot charcoal reacts with oxygen, which burns the carbon, turning it into carbon dioxide; that reaction between charcoal and oxygen is what you perceive as the glowing embers in a fire. The charcoal glows brightly because it releases large amounts of energy when it bonds to oxygen. There are also some white minerals left behind after the charcoal burns, which is often called ash.
If you instead heat the wood in a closed container with a small vent, the smoke displaces most of the oxygen in the container; it is very important to have a vent in the container, which allows the excess smoke to escape so that pressure does not build until the container explodes. Because the oxygen has been displaced, the hot charcoal that is left behind in the container does not have oxygen with which to react, therefore, it does not burn. Once the container is allowed to cool completely, the only thing that is left in the container is charcoal.
The Completed Cube
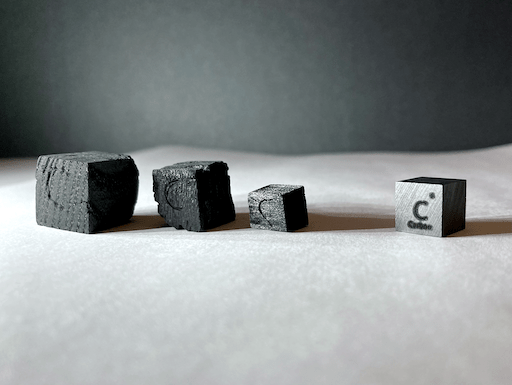
I made a few charcoal cubes, which, if you ask me, don’t look too bad for being made by fire and a file. Due to the fact that charcoal has some ash trapped in its structure, these charcoal cubes are likely 70% to 80% carbon, compared to the graphite cube from Luciteria, which is 99.9% carbon.
Another interesting characteristic about my charcoal cubes is that their densities are much less than that of the graphite cube. The graphite cube weighs about 1.8 grams, but the largest charcoal cube weights about 1 gram, and the smallest one weighs less than 0.1 grams, which means that these three charcoal cubes combined weigh about the same amount as the graphite cube!
If you wish to have your own carbon cube, you could make your own charcoal cube, because this is one of the cheapest, safest, and easiest element cubes to make, but if you don’t wish to make your own, you can always buy one from Luciteria here.
Do you have any recommendations for which element cube to make next?

